Aug 10, 2021
Your impact: How FBC funded research is leading the way to new treatments for retinal degeneration
Until recently there were no treatments for inherited retinal diseases (IRDs) but investments in research over the past decades have started to pay off. Treatments are now being tested and, in the case of the gene therapy called Luxturna, are even being approved for use.
Gene therapies have had the most success to date as a treatment for IRDs. However, this approach is limited by the fact that a new gene therapy must be developed for each different gene or gene mutation which is time-consuming, expensive, and not always possible.
That is why scientists are also exploring other strategies to develop treatments to preserve and restore vision, including new drug therapies, stem cell replacement, optogenetics, and even artificial retinal implants. These treatments could be considered “universal” treatments that improve vision for retinal diseases caused by many different gene mutations or non-genetic diseases like age related macular degeneration.
Thanks to continued support from Fighting Blindness Canada donors, we are funding scientists to develop these universal therapy strategies, including Dr. Michel Cayouette who is trying to improve stem cell therapy, and Dr. Bremner who is identifying new drug targets to reduce photoreceptor cell death. Keep reading to learn more about each of their projects.

Dr. Michel Cayouette (IRCM, Montreal): FINDING BETTER WAYS TO MAKE PHOTORECEPTOR CELLS
Stem cells are a special type of cell that can make many different kinds of cells. In stem cell therapy, new photoreceptor cells are created in a lab from stem cells and put back into the eye replacing photoreceptor or other retinal cells that have been damaged or died. Stem cell therapy holds the promise of being able to restore vision even after retinal degeneration has progressed.
Despite recent research advances, stem cell therapies still face major challenges. One, that Dr. Cayouette is trying to solve, is that scientists are not able to effectively make a type of photoreceptor called cone cells. There are two types of photoreceptor cells, rods and cones. Rod cells are responsible for night and peripheral vision while cone cells are important for detail, daytime and central vision. Currently, scientists can make rod cells more easily than cone cells and figuring out how to make more cone cells will be critical to developing a successful stem cell therapy.
Why is it so difficult to make cone photoreceptor cells? Dr. Cayouette’s hypothesis is that retinal stem cells can only make cone cells at a certain point in their development and once they are past this point it’s too late.
The goal of this project is to uncover how stem cells regulate the timing of cone photoreceptor production. Dr. Cayouette hopes that by discovering this mechanism, scientists can manipulate stem cells and force them to produce more cone photoreceptor cells for cell replacement therapy.
Dr. Cayouette’s team is using a technique called gene editing to “delete” genes and study what that gene does. Using this technique, Dr. Cayouette found that when two genes, Pou2f1 and Pou2f2 (Pou2f1/2) were deleted, mouse retinal stem cells made less cone cells. Conversely, when more Pou2f1/2 were put into stem cells, more cone cells were produced.
This work was published in the high impact journal Development in 2020 and was picked as a Research Highlight because of its potential impact to the vision research field.
Access the interview with Dr. Cayouette and his PhD student Awais Javed.
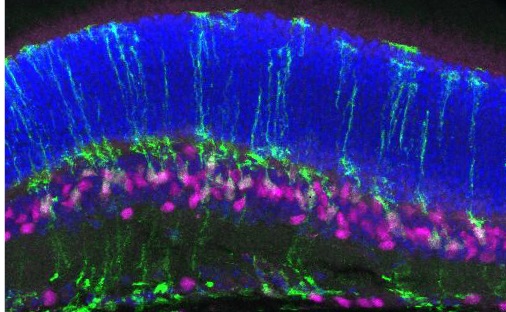
This project is in the final year of FBC funding and Dr. Cayouette and his team will be taking what they have learned and expanding on this exciting project. One avenue they are exploring is if the Pou2f1/2 genes can turn non-light sensing retinal cells into cone photoreceptors. In many cases of retinal degeneration, photoreceptor cells are lost, but the other retinal cells are healthy and retain their connections with the optic nerve and the brain. If these other retinal cells can be turned into light sensors, it would bypass one of the challenges of cell replacement, ensuring the new cells integrate in the right place and form the right connections so that they can pass on light signals.

Dr. Rod Bremner (Lunenfeld-Tanenbaum Research Institute, Toronto): REDUCING PHOTORECEPTOR CELL DEATH.
Photoreceptor cell death is the primary cause of vision loss in many IRDs like retinitis pigmentosa (RP) or Stargardt disease. There are currently no cures for these diseases, which means that once photoreceptors start to die a person’s vision will progressively worsen. Dr. Bremner is trying to change this by looking for ways to stop photoreceptor cell death.
Genes provide instructions that help cells function. If the instructions are damaged by a gene mutation the cell’s function or ability to survive is compromised. During a retinal degenerative disease, gene mutations cause so much damage to photoreceptor cells that the cell turns on “death” genes that pushes a cell to die.
In order to identify which genes are “death” genes, Dr. Bremner is using a technique called gene knockdown to block the function of specific genes. If a gene that causes cell death is blocked, the cell won’t be able to die. Dr. Bremner’s team is using an animal model of RP where most of the rod photoreceptor cells die within a few weeks after birth. However, if a gene that causes death is blocked, more rod photoreceptor cells will survive.
Dr. Bremner is using a “high-throughput” screening approach so that he can test thousands of genes at the same time. This will save time, rather than looking at each gene one at a time, and increase the chance that multiple death genes will be identified at the end of this project.
During the first year of Dr. Bremner’s FBC-funded project, his team set up and tested the high-throughput technology, to ensure that it was working properly and consistently.
Dr. Bremner’s team performed two test runs of the screen. As hoped, they saw that the retinas from mice that had gene knockdowns had more photoreceptor cells than retinas that weren’t treated. Based on this, the team is confident that the high-throughput screen is working properly. In the second year of this project, the team will repeat these experiments multiple times to make sure the results are consistent, as well as performing these experiments in other genetic models of RP.
In the long term, Dr. Bremner will test drugs against any identified death gene to find pharmacological ways to prevent cell death. Dr. Bremner has focused on genes whose function can be blocked by FDA-approved drugs. Since the drugs are already FDA-approved the goal is to shorten the time towards a potential clinical trial.
To learn more about other FBC-funded research, visit fightingblindness.ca/research/fbc-funded-research/.

Join the Fight!
Learn how your support is helping to bring a future without blindness into focus! Be the first to learn about the latest breakthroughs in vision research and events in your community by subscribing to our e-newsletter that lands in inboxes the beginning of each month.

