May 5, 2020
Improving AMD Treatments by Studying Cell Movement

Age-related macular degeneration (AMD) is the leading cause of vision loss in people over the age of 50, affecting approximately 1.4 million Canadians. There are two kinds of AMD: dry AMD which is more common and less severe and wet AMD, where blood vessels grow abnormally and can leak blood and fluid into the eye. This damages cells called retinal pigment epithelial (RPE) cells, which provide critical support to the light sensing cells in the retina called photoreceptors cells. Because RPE cells play such an important role, their damage and loss can cause severe vision loss.
The main treatment for wet AMD are anti-VEGF treatments which reduce the abnormal growth of blood vessels. Unfortunately, this treatment can stop working after a while or may not work for some individuals at all. In addition, once RPE cells have been lost, it can be hard to restore vision. That’s why scientists are looking at other types of treatments, including cell replacement therapy, which you may also know as stem cell therapy. In cell replacement therapy healthy RPE cells are transplanted into the eye to replace damaged cells. In order to be successful, these transplanted RPE cells have to move from the site of injection to the sites of RPE damage, and once there have to carry out normal RPE cell function.
This is where Dr. Sarah McFarlane’s FBC funded research comes in. She is studying how RPE cells move in a healthy eye after injury or during a disease like AMD. As Dr. McFarlane says, “one of the reasons why there are no treatments for many blinding eye diseases is because we do not know enough about how the different cells implicated in disease behave and interact.” Cell replacement therapy for AMD is just starting to be tested in clinical trials and research like this will be important to improve therapies and increase the chance that they are successful.
Dr. McFarlane work uses a gene editing technique called CRISPR to study the role of different genes in a zebrafish animal model. Here is some information about the CRISPR technique and zebrafish:
Using CRISPR to Edit Genomes
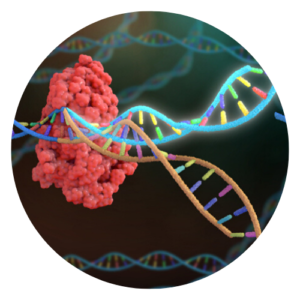
CRISPR is the name of a technique that is used to change the DNA sequence of a gene. CRISPR is made up of different molecules but it is essentially a pair of molecular scissors that cuts DNA at a specific point, puts a new DNA sequence in, and then “sews” the cuts up to create a new piece of DNA. CRISPR is a powerful tool that scientists can use to change a gene’s function, including turning it off, introducing a mutation to study what this does to the cell, or fixing a mutation to recreate a functional gene.
Zebrafish: An Important Animal Model to Study Eye Disease

You may know that scientists use animals such as mice to study diseases to find out what a gene mutation does or test new drugs. What many people don’t know is that other animals are also important for research, including fruit flies, nematode worms, and zebrafish. Zebrafish are small fish that are part of the minnow family. They are a useful model to study eye diseases because they are less expensive, smaller, and easy to grow. Their eyes also have a similar structure and function as human eyes.
With support from FBC, Dr. McFarlane has been trying to identify the signals that control RPE cell movement. Dr. McFarlane’s team used CRISPR to “cut out” the genes of potential signal molecules and then examine if the cells could still move properly. Excitingly, they found that molecules that are known to control the movement of nerve endings also controlled the movement of RPE cells. As Dr. McFarlane explains, “By understanding how the RPE cells move and function in a healthy situation, we can start identifying mechanisms to get these cells to move to areas of RPE death in disease or damaged retina, and to maintain photoreceptor health once there.” The team is now testing if these molecules play a role in how RPE cells respond after injury (such as in AMD).
These results will provide important information to scientists who may be able to find ways to turn these control signals on and off and influence where RPE cells go after they are injected during cell replacement therapy. Dr. McFarlane’s study has made great progress thanks to generous donations to FBC, and we are excited to see where this research leads!
Join the Fight!
Learn how your support is helping to bring a future without blindness into focus! Be the first to learn about the latest breakthroughs in vision research and events in your community by subscribing to our e-newsletter that lands in inboxes the beginning of each month.

