May 1, 2018
RNA Therapies for Inherited Retinal Diseases (IRDs)
I recently participated in a tremendously inspiring meeting, hosted by ProQR, which is a company that is creating new treatments for people living with rare diseases, including blinding eye diseases. ProQR specializes in developing RNA therapeutics, and I was there to learn about a new clinical trial that they are planning to treat the inherited blinding eye disease Usher syndrome.
Usher syndrome is the leading cause of combined deafness and blindness. ProQR is currently developing two new drugs for different mutations that cause Usher syndrome type 2. These new drugs are made of short pieces of RNA (hence the name, RNA therapeutics).
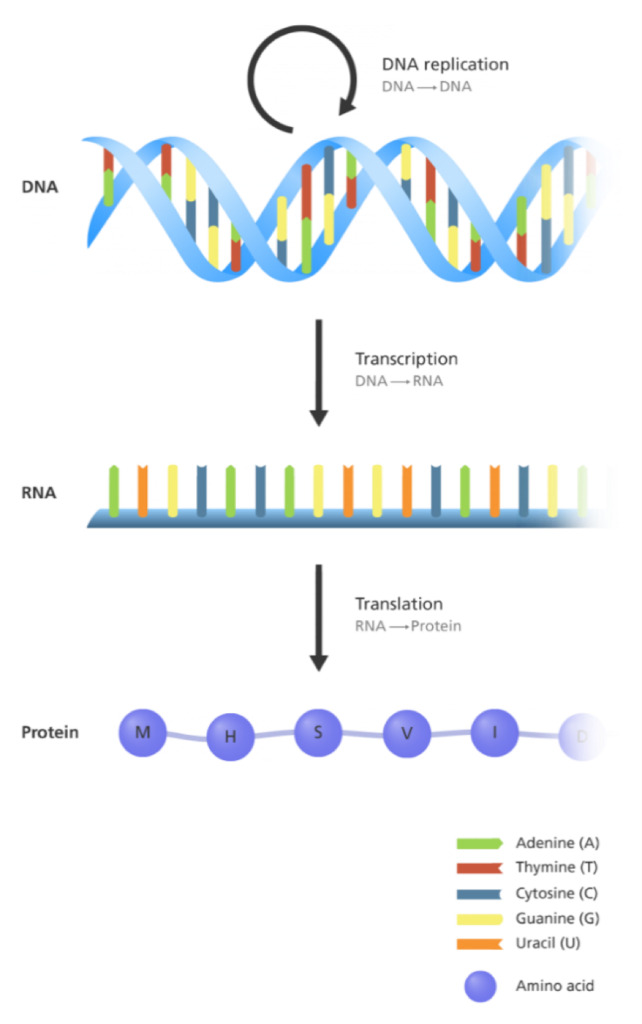
Before I can describe how RNA therapeutics work, it’s first important to remember “the central dogma of biology,” which explains the flow of genetic information from DNA to RNA to protein (please see the central dogma image below). The central dogma provides an overly simplified, but helpful, description that shows the connections between DNA, RNA, and proteins. Basically, DNA makes RNA and RNA makes proteins. Ultimately, it’s the proteins that are needed to make your body work. Inherited blinding eye diseases, like Usher syndrome, are caused by genetic mutations. These genetic mutations can be treated by fixing the DNA (this is how gene therapy works), or, by fixing the RNA (this is how RNA therapy works).
Whereas gene therapies are a one-time treatment that deliver a replacement gene at the DNA level, RNA therapies are delivered as a kind of drug that works by targeting a very specific part of the damaged RNA. The RNA therapy that ProQR is developing will work for Usher syndrome patients who have a mutation in exon 13 of the USH2A gene.
At the ProQR meeting in the Netherlands, I was fortunate to meet an international group of Usher syndrome patient representatives from different organizations in the USA, Canada, UK, Ireland and the Netherlands. Together, we shared insights and experiences to inform the development of ProQR’s upcoming clinical trial for patients living with USH2A, which is expected to start before the end of year. I left the meeting feeling inspired about ProQR’s progress and its ongoing commitment to include patients right from the very beginning!
The work of ProQR is only possible because of the research developments that have been made, thanks, in part, to research funded by Fighting Blindness Canada (FBC). Additionally, the Foundation Fighting Blindness in the United States, is a key driver and funder of ProQR’s clinical program. To me, the involvement of patients and patient groups right from the very beginning is what makes these developments so inspiring. We can all celebrate progress together, and know that investing in vision research is the only way to end blindness!
Join the Fight!
Learn how your support is helping to bring a future without blindness into focus! Be the first to learn about the latest breakthroughs in vision research and events in your community by subscribing to our e-newsletter that lands in inboxes the beginning of each month.
